Explain Why Water Is Polar | Water interacts differently with charged and polar substances than with nonpolar substances because of the polarity of its own molecules. It is composed of one oxygen atom and two hydrogen atoms. Water ( h2o ) is polar because of the bent shape of the molecule. The two hydrogen atoms and one oxygen atom within water molecules (h2o) form polar covalent bonds. They spend more time around the o than with the h atoms.
It also acts as a polar solvent. Water has a simple molecular structure. Water is a polar molecule. Water (h2o) is polar because of the bent shape of the molecule. Thus, polarity is the unequal sharing of charge of the molecule.
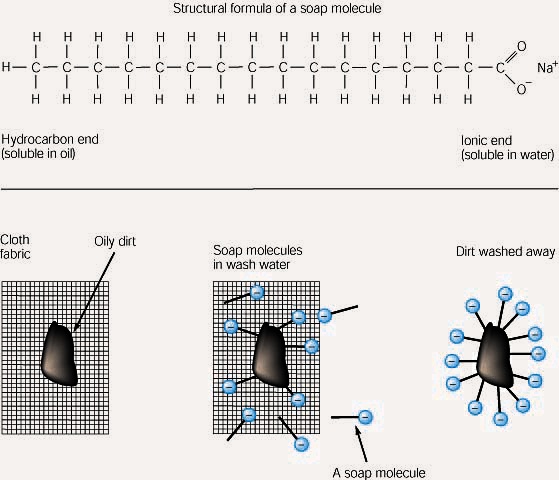
Water ( h2o ) is polar because of the bent shape of the molecule. Polarity of a water molecule. It also acts as a polar solvent. Water has a simple molecular structure. This shows that electrons are more attracted to the . It is composed of one oxygen atom and two hydrogen atoms. Point out that the electron cloud around the oxygen is darker than the electron cloud around the hydrogen. How many hydrogen bonds can each water molecule form? Water interacts differently with charged and polar substances than with nonpolar substances because of the polarity of its own molecules. It has one side that is positively charged and one side . This property is called electronegativity. The two hydrogen atoms and one oxygen atom within water molecules (h2o) form polar covalent bonds. The shape means most of the negative charge from .
Polarity is the unequal sharing of charge of the molecule. It also acts as a polar solvent. Thus, polarity is the unequal sharing of charge of the molecule. It has one side that is positively charged and one side . While there is no net charge to a water .
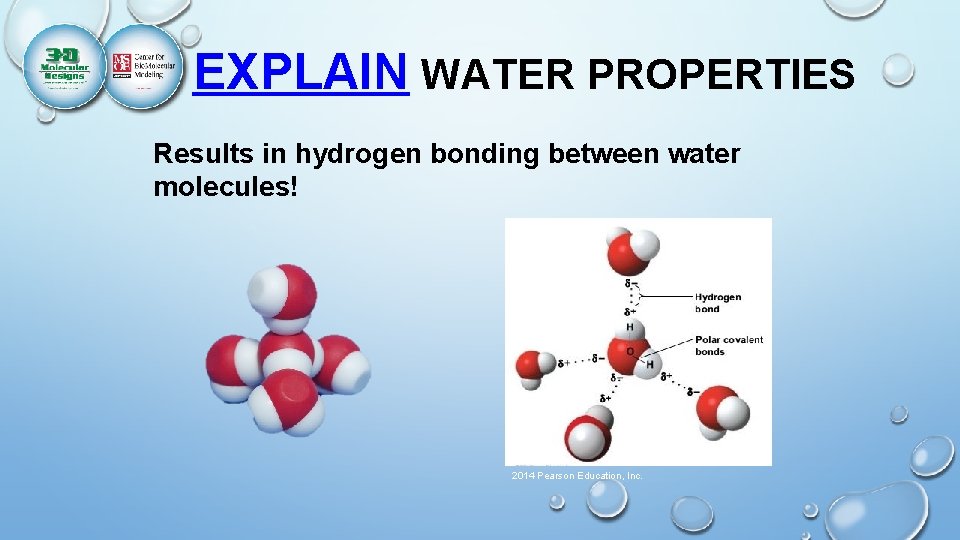
The two hydrogen atoms and one oxygen atom within water molecules (h2o) form polar covalent bonds. They spend more time around the o than with the h atoms. Polarity is the unequal sharing of charge of the molecule. The shape means most of the negative charge from . It has one side that is positively charged and one side . Point out that the electron cloud around the oxygen is darker than the electron cloud around the hydrogen. Water has a simple molecular structure. The electrons in the covalent bond between the o and h atoms aren't shared equally. While there is no net charge to a water . Water (h2o) is a polar molecule because the electrons of the hydrogen atoms get pulled towards the electrons of the oxygen atom. It also acts as a polar solvent. How many hydrogen bonds can each water molecule form? This property is called electronegativity.
Water is a polar molecule. Water (h2o) is polar because of the bent shape of the molecule. Water ( h2o ) is polar because of the bent shape of the molecule. Polarity is the unequal sharing of charge of the molecule. Point out that the electron cloud around the oxygen is darker than the electron cloud around the hydrogen.

It also acts as a polar solvent. Water ( h2o ) is polar because of the bent shape of the molecule. How many hydrogen bonds can each water molecule form? Water (h2o) is a polar molecule because the electrons of the hydrogen atoms get pulled towards the electrons of the oxygen atom. Point out that the electron cloud around the oxygen is darker than the electron cloud around the hydrogen. The electrons in the covalent bond between the o and h atoms aren't shared equally. Water (h2o) is polar because of the bent shape of the molecule. Water interacts differently with charged and polar substances than with nonpolar substances because of the polarity of its own molecules. They spend more time around the o than with the h atoms. This shows that electrons are more attracted to the . Water is a polar molecule. Polarity of a water molecule. It is composed of one oxygen atom and two hydrogen atoms.
Explain Why Water Is Polar: It is composed of one oxygen atom and two hydrogen atoms.
No comments:
Post a Comment